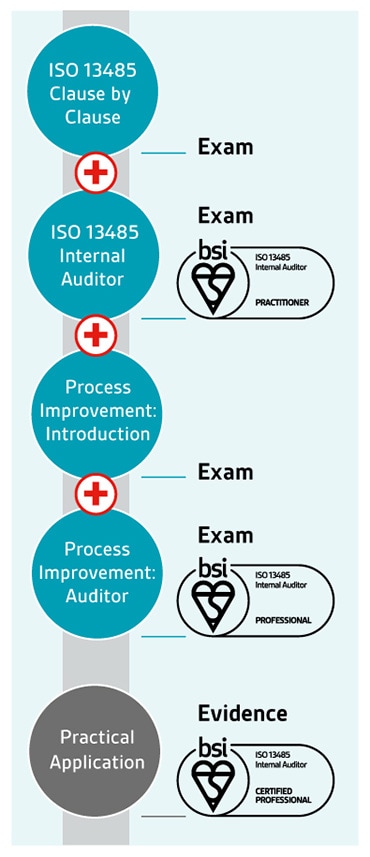
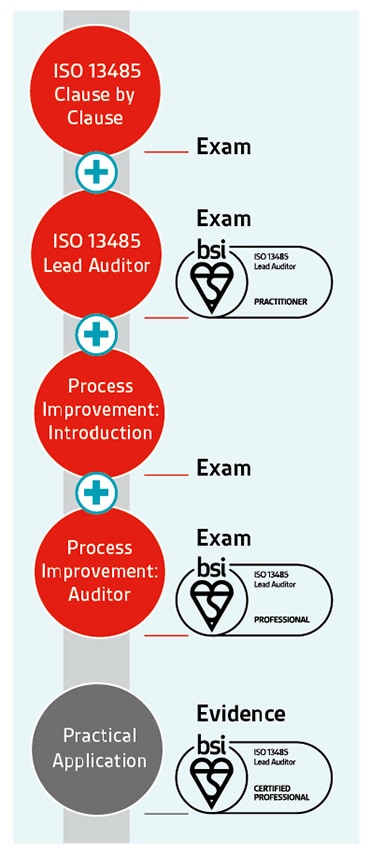
The ability to identify opportunities for improvement is an important skill for an internal or lead auditor. It could involve a reduction in incidents, improved processes or increases in performance.
That's why we've developed auditor qualifications for ISO 13485 – medical devices quality management systems - to give you the skills and knowledge you need to be an auditor practitioner, and by teaching you techniques in process improvement to become an auditor professional.
Why take your ISO 13485 Auditor Qualification with BSI?
- BSI delivers over 50,000 auditor training courses a year
- BSI's own auditors deliver over 200,000 auditor assessment days a year
Once you've achieved your professional qualification, you can apply to become certified – a 3-year rolling programme to validate the practical application of your learning and your continuing professional development within your industry.