Devices covered by AIMDD/MDD certificates from 26 May 2021
News: 10 June 2021
The Medical Devices Regulation (MDR) EU 2017/745 came into effect on 26 May 2021. Article 120 of the MDR has specific transitional provisions in relation to devices that continue to be placed on the market under the Active Implantable Medical Devices Directive (AIMDD) 90/385/EEC or the Medical Devices Directive (MDD) 93/42/EEC and for certificates issued under those legislation.
We would like to provide additional guidance on two specific topics related to Article 120 of MDR:
- reporting changes for devices that continue to be certified under AIMDD and MDD;
- and changes to AIMDD and MDD certificates issued by BSI The Netherlands (NB 2797) from the 26 May 2021.
Reporting changes to the notified body for devices covered by AIMDD/MDD certificates from 26 May 2021
MDR Article 120.3 states that devices covered by valid AIMDD/MDD certificates may continue to be placed on the market or put into service until 26 May 2024 as long as they continue to meet the requirements of those legislation and there are no significant changes in their design or intended purpose. Please refer to MDCG 2020-3 for additional guidance on what constitutes a significant change in design or intended purpose.
For these devices, manufacturers need to adapt/update their change reporting processes and procedures to meet both the MDR transitional provisions and the change reporting requirements under the applicable conformity assessment annexes of the relevant legislation (AIMDD or MDD). Updates to the processes/procedures and their implementation will be checked during routine BSI surveillance audits. In cases where it is unclear as to whether a proposed change is allowed to be carried out under AIMDD/MDD or not, it is recommended that the manufacturer reach a preliminary conclusion based on their analysis and submit this to the Notified Body (via the MDF4900 Change Notification Form) for confirmation prior to implementation of the change.
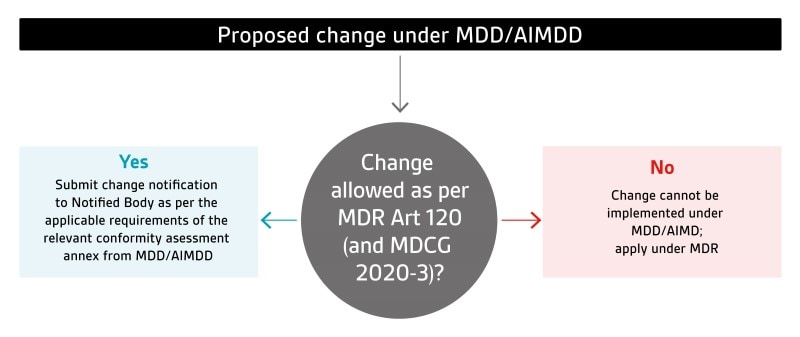
Changes to AIMDD/MDD certificates issued by BSI NL (NB 2797) from 26 May 2021
MDCG 2020-3 states that notified bodies are not allowed to issue new AIMDD/MDD certificates or modify/amend/supplement existing certificates from 26 May 2021. It is suggested that notified bodies confirm in writing any changes that have been reviewed and approved from 26 May 2021 and that such written confirmation corrects or complements information on an existing certificate issued prior to 26 May 2021.
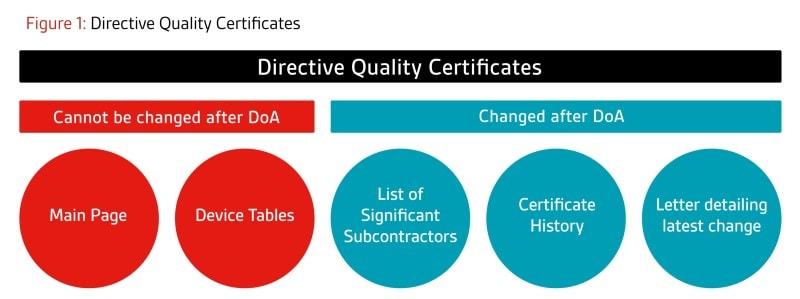
Following the above guidance, BSI will not be making any changes to the main certificate pages of AIMDD/MDD certificates from 26 May 2021. Any changes reviewed and approved will be communicated in the following way:
- Certificates based on quality system annexes (e.g. full quality assurance certificates):
The main certificate will remain unchanged. Supplementary pages such as the Significant
Subcontractor page and History page will continue to be updated as required, as these are not
considered part of the main certificate. In addition, a letter will be issued at the end of each project
describing the latest change(s) approved as a part of that project.
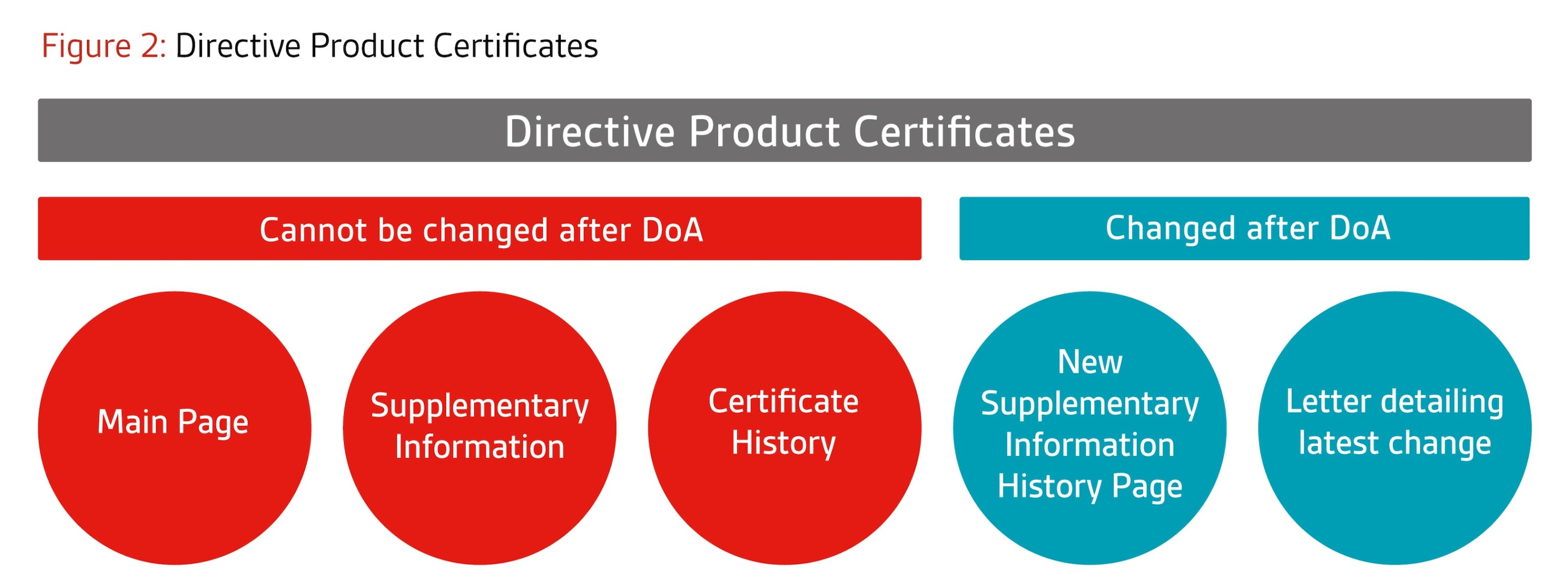
- Certificates based on product annexes (e.g. design exam certificates): The main certificate
will remain unchanged. A new supplementary page will be issued to capture the change history. In
addition, a letter will be issued at the end of each project describing the latest change(s) approved as
part of that project.
Other information
It is also important to remember that for all devices placed on the market until 26 May 2024 or put into service until 27 May 2025 under AIMDD/MDD, the requirements of MDR relating to post-market surveillance, market surveillance, vigilance, registration of economic operators and of devices (pursuant to availability of EUDAMED) shall apply in place of the corresponding requirements in those Directives. These requirements from MDR will apply even if such devices are not being transitioned to MDR. Implementation of these requirements by the manufacturers will be checked during routine BSI surveillance audits.
Who can I contact for further information?
If you have questions or concerns, we recommend using the free material referenced in the first instance. Your BSI Scheme Manager can provide further information if you still have questions.


