Preparing your submission for the Expert Panels of the EC
News: 26 May 2021
On 1 April 2021, the European Commission (EC) launched its Expert Panels to provide consultation in relation to activities defined in Article 54 of the Medical Devices Regulation (MDR EU 2017/745), also known as the Clinical Evaluation Consultation Procedure (CECP).
Class III Implantable and Class IIb Rule 12 active devices intended to Administer or Remove a Medicinal Substance (ARMS) may be subject to CECP. Exemptions to this process are listed in Article 54, Paragraph 2. Modifications to legacy devices that go beyond the requirements of the MDR will be subject to CECP.
In the absence of EUDAMED, the EC has made available an alternative mechanism via the CIRCABC platform for conducting the CECP process.
Manufacturers of Rule 12 Class IIb active ARMS devices should be aware that all devices in the generic device group may have to undergo CECP. There will be a partial review of the Technical Documentation, particularly the clinical evaluation, even if the device hasn’t been chosen as a representative device for the generic device group to confirm if CECP is required. Please refer to MDCG 2019-13 for further guidance on this aspect.
Required Documentation
For those medical devices that will be sent for the consultation procedure, the following documentation is required to be submitted for consultation:
- The Clinical Evaluation Assessment Report (CEAR)*
- The Clinical Evaluation Report (CER)
- The Clinical Evaluation Plan
- The Post Market Clinical Follow Up (PMCF) Plan
- The Post Market Clinical Follow Up (PMCF) Evaluation Report (where applicable)
*Please note that the CEAR is created as part of the notified body’s conformity assessment and is not part of the manufacturer’s Technical Documentation.
It is important to note that the notified body can only submit the documents listed above. If, during the conformity assessment process, it is identified that the Technical Documentation requires an update of information (e.g. the CER), this must be completed before submission for CECP.
Document Markings
At the instruction of the EU Commission, the notified body must ensure that the documentation to be shared with expert panels is appropriately marked.
Please ensure that each of your documents specified above for panel submission has the following two markings on the front page:
1) A security marking: SENSITIVE to indicate that the information is Sensitive Non-Classified
2) A distribution marking: RELEASABLE TO: EXPERT PANELS (EXPAMED) to indicate restrictions on the authorized recipients.
- The security marking must be in Times New Roman, font size 14, bold, and capital letters.
- The distribution marking must be provided in the next line following the security marking and printed in Times New Roman, 14, italics.
An example is given below (D5.2 v1.1 Instructions for Notified Bodies, EU 2021).
SENSITIVE
RELEASABLE TO: EXPERT PANELS (EXPAMED)
PMCF Plan and Evaluation Report Templates
The PMCF Plan and, where applicable, PMCF Evaluation Report, should follow the templates provided by the EU Commission.
The templates will allow the expert panels to navigate the documents to locate the relevant information. These templates can be downloaded here:
- MDCG 2020-7 provides the template for PMCF Plan
- MDCG 2020-8 provides the template for the PMCF Evaluation Report (where applicable)
Please note: if alternative templates are used, the screening panels at the EU Commission may reject the submission. Please ensure all headings and information in your PMCF Plan and PMCF Evaluation Report align with the MDCG guidance documents.
The CECP Process
The CECP process can only be initiated when all other consultations, if applicable (e.g. medicinal), are complete.
The CECP process will go through two phases:
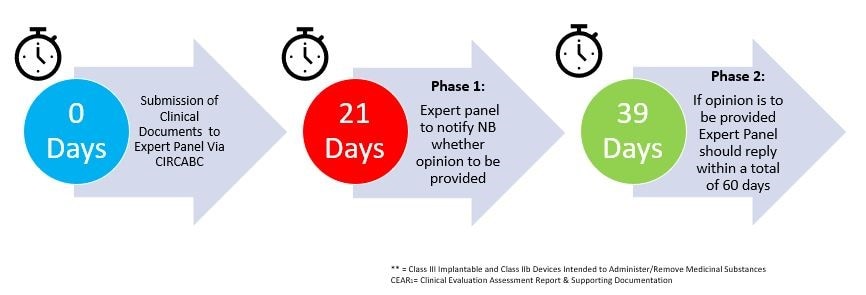
Phase 1 – Screening Panel – To decide whether the Expert Panel intends to provide an expert opinion
Phase 1 is expected to take 21 days. During this stage, the screening panel will evaluate the documentation
to determine whether or not to provide a scientific opinion. Devices that are determined to meet the criteria
will be subject to Phase 2. If the screening panel choose not to provide an opinion, the notified body can go ahead with its usual certification procedures.
Phase 2 – Expert Panel Opinion
Phase 2 is expected to take a further 39 days. During this stage, a group of expert panellists in the relevant medical discipline will deliver their opinion to the notified body. The notified body will consider the opinion of the Expert Panel as part of its final evaluation, which may lead to further actions. The notified body may not have the opportunity to answer questions or clarify areas to the Expert Panels, and therefore it is critical that manufacturers adhere to the guidance provided.
Further Information
A BSI Technical Documentation Completeness Checklist is currently being updated to assist manufacturers in marking up documents for those devices potentially subject to CECP.
Further information about the EU Expert Panels and the decision criteria on whether an opinion will be required is available on the European Commission web page.


