Passionate about patient safety
Our mission is to ensure patient safety whilst supporting timely market access to medical technology in a sustainable manner. We strive to set the global standard through conducting impartial, responsive, robust and thorough conformity assessments, evaluations and certifications that are recognized and trusted worldwide.
BSI The Netherlands (2797) is a leading Notified Body; we review medical devices to ensure that they conform to the requirements of the European Directives and Regulations. BSI UK (0086) is a UK Approved Body able to provide conformity assessments under the new UKCA scheme.
Maintaining quality and delivering excellence
BSI Medical Devices offers certification services to support your global market access goals. We are:
- A designated EU Notified Body
- A UK Approved Body
- An accredited ISO 13485 Certification Body
- A recognized Auditing Organization under the Medical Device Single Audit Program (MDSAP)
- A recognized Certification Body in many global markets
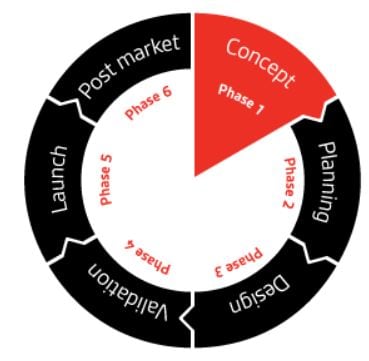 Supporting you throughout the product lifecycle
Supporting you throughout the product lifecycle
Regardless of which stage you are at, BSI can work with you to minimize your corporate risk and ensure you remain compliant.
Talk to us early to take advantage of our full range of services.