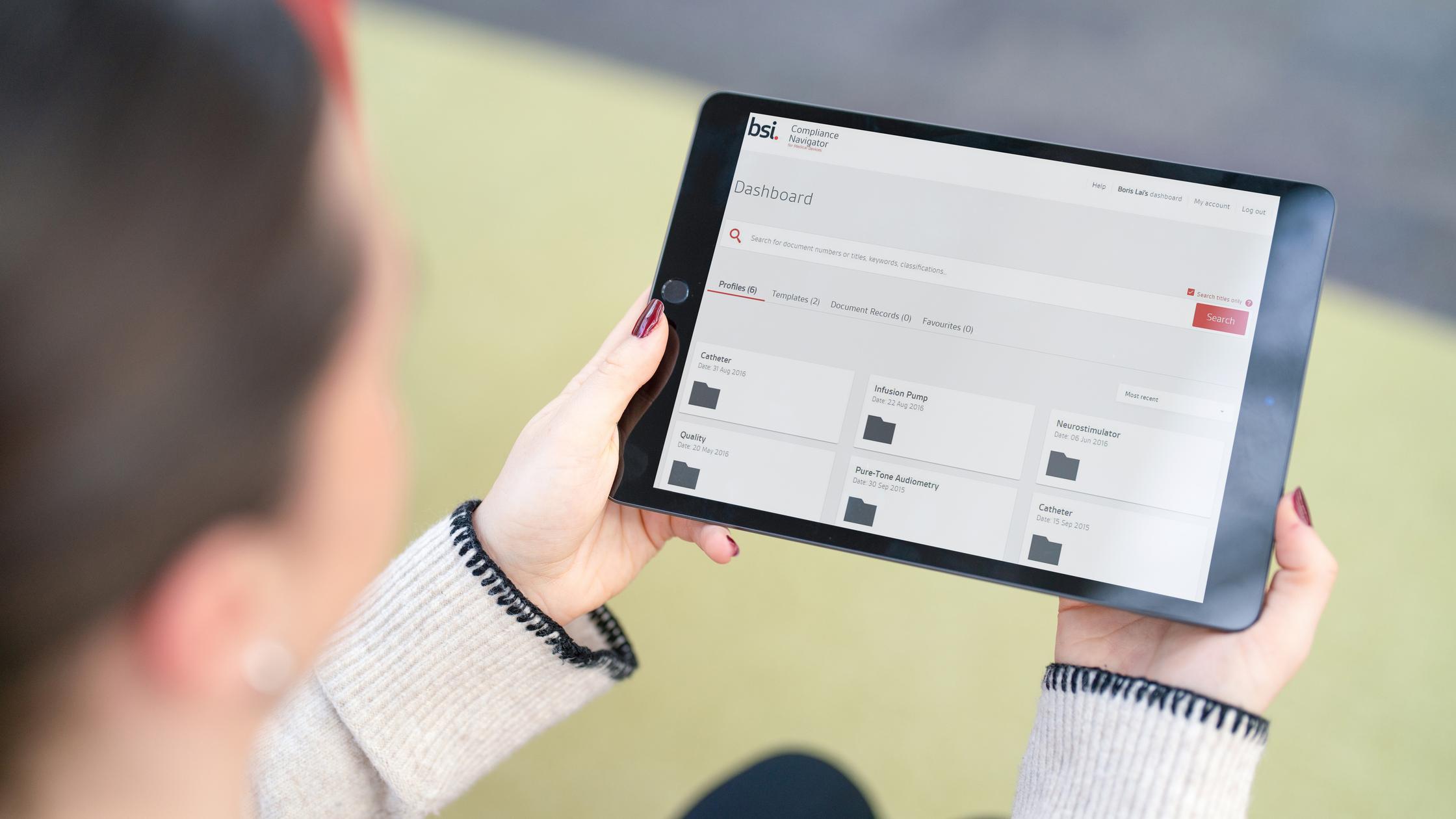
However, keeping abreast of new and updated standards and regulations is complex and time-consuming – and an error can result in failure to launch, unforeseen costs, delays and even product recalls.
Compliance Navigator is an online tool that helps manufacturers discover, organize and respond quickly to changes in the regulatory landscape and mitigate these risks.