MHRA on UKCA Standstill Period
Date: 01 November 2022
On 21st October 2022, the MHRA officially announced a twelve-month extension to the current standstill period to comply with UKCA marking regulations. From 1st July 2024, legislative transitional arrangements will apply for placing all Medical Devices and In-Vitro Diagnostic Medical Devices on the Great Britain market. Please see the revised transitional timeline below (subject to change):
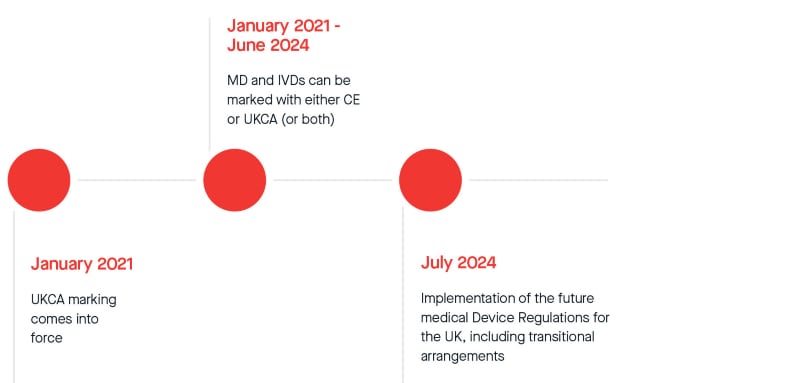
Despite the possible reassurance brought by this extension, we strongly urge you to continue to submit your signed proposal and submit relevant set of fully compliant technical documentation as soon as possible to minimize the risk of not completing the work in a timely manner. We will schedule and conduct reviews at the earliest opportunity based on availability of reviewers. Please be assured we are focusing on the capacity of our reviewers to be able to meet the demands for certification as efficiently as possible.
Our priority remains to maintain patient safety and ensure compliant reviews for all products within the current and future regulatory frameworks.
If you require additional information relating to your UKCA submission, please contact your Scheme Manager.
Further guidance can be found at GOV.UK and on our UKCA dedicated webpage.
Yours sincerely,
Vishal Thakker
Head of UK Approved Body & Senior Regulatory Lead, Regulatory Services (Medical Devices)
