BSI Electronic Client Portal
We are changing how documentation is uploaded to the BSI Electronic Client Portal which is used by BSI clients to upload vigilance reports and technical documentation. The portal will include additional functionalities to allow manufacturers to upload individual documents as required for EUDAMED.
- Manufacturers will be able to submit SS(C)P documents against certificate number(s) while specifying the Basic UDI-DI(s) covered by the SS(C)P documents. Please do not submit translations of SS(C)P documents until BSI sends notification that uploads are starting to EUDAMED.
- Manufacturers will be able to submit a PSUR document against certificate number(s) while specifying the Basic UDI-DI(s) covered by those PSURs. PSURs for class III, implantable and
class D devices should be submitted via the portal until EUDAMED is available for submission.
Manufacturers can access the instructions on how to submit these documents once they are logged in to BSI Electronic Client Portal.
MDR/IVDR Certificates - Four-date format
Moving forward, all BSI IVDR and MDR certificates will detail four dates:
- First Issue Date
- Current Issue Date
- Starting Validity Date
- Expiry Date
This change will be implemented for any MDR/IVDR certificates issued or re-issued henceforth. The four-date format is needed to align with the requirements when registering certificates in EUDAMED.
The table below shows a comparison of the current three-date format and the new four-date format: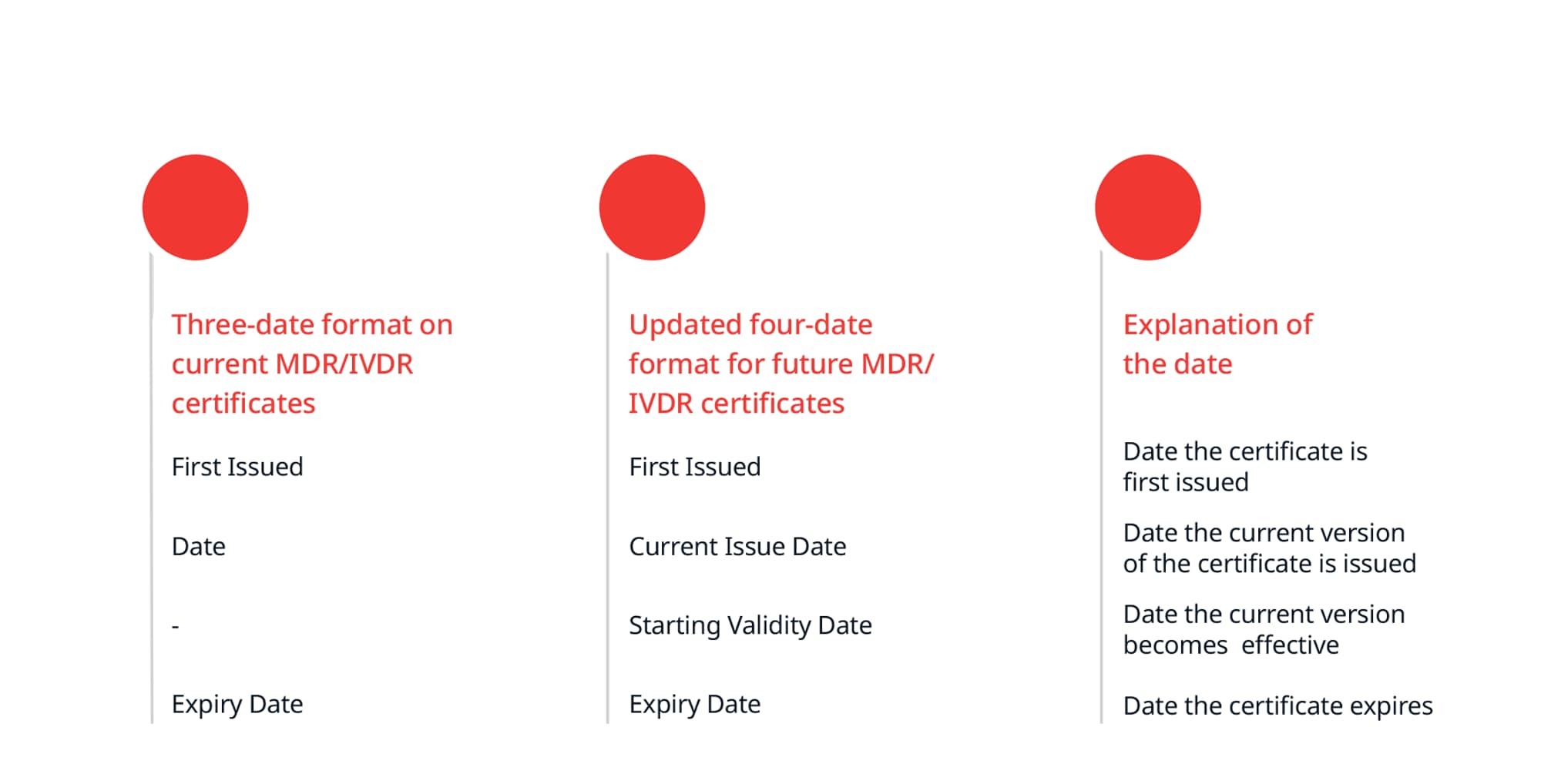 MDR/IVDR certificates already issued with the three-date format continue to remain valid and will be converted to the four-date format when reissued (for any reason).
MDR/IVDR certificates already issued with the three-date format continue to remain valid and will be converted to the four-date format when reissued (for any reason).
This change does not affect UKCA and EU Directive certificates, which will continue to use the three-date format.
Critical subcontractors and crucial suppliers
Current BSI issued quality system annex based MDR/IVDR certificates, such as Annex IX Chapter I & III certificates, include supplementary page(s) that list the critical subcontractors and crucial suppliers associated with the products covered by the scopes of those certificates. To allay any confidentiality concerns, MDR/IVDR certificates, moving forward will not include this supplementary information on subcontractors/suppliers. Instead, BSI will maintain this information within internal records.
There is no change to the process of approving any changes to critical subcontractors/crucial suppliers itself. Manufacturers must continue to notify BSI of any plans to make changes to their critical subcontractors and crucial suppliers during the certificate validity.
All quality-system based MDR/IVDR certificates issued or re-issued henceforth will not include the subcontractor/supplier information as described above. Current MDR/IVDR certificates already issued with this information continue to remain valid and will be transitioned to not having the subcontractor/supplier information the next time they are reissued (for any reason).
BSI will also implement the above principles to UKCA and EU Directive certificates moving forward to maintain a harmonised approach across all the legislations.
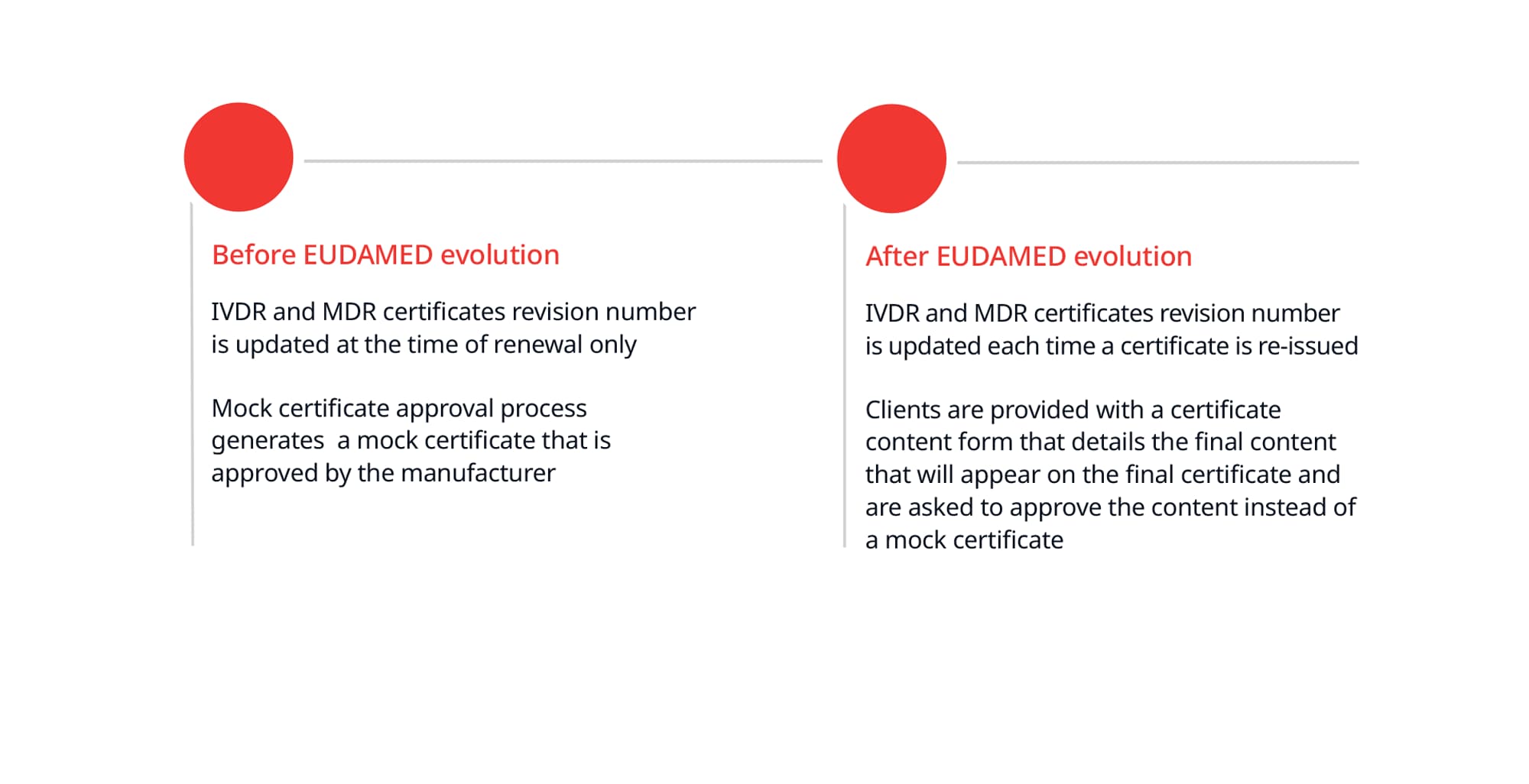
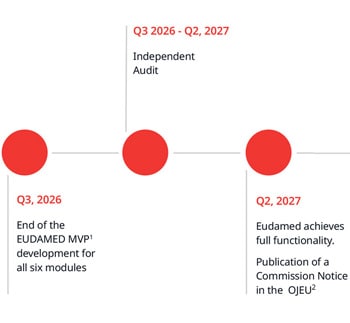
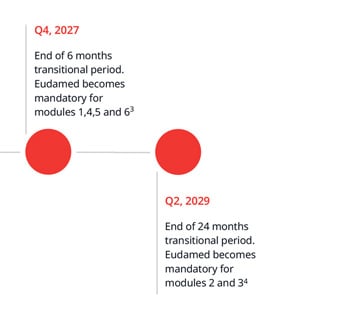
Other anticipated changes to BSI certificates and approval process
The changes below are anticipated to be implemented over the next few months before BSI starts submitting information into EUDAMED.




 MDR/IVDR certificates already issued with the three-date format continue to remain valid and will be converted to the four-date format when reissued (for any reason).
MDR/IVDR certificates already issued with the three-date format continue to remain valid and will be converted to the four-date format when reissued (for any reason).



