MHRA's consultation response on future UKCA legislation
Date: October 17, 2022
We have received a number of queries regarding MHRA’s consultation response on future UKCA legislation – so we wanted to get in touch to clarify the current situation.
The MHRA consultation response indicates the UK Government’s thinking on the direction of the future legislation – but to be very clear; it is not definitive and not legal yet.
As part of the response to the consultation, some key points were addressed including potential transitional provisions (see below). As you can see, the proposed transitional timelines may provide some breathing room and may particularly benefit some manufacturers, especially those who have completed their MDR, IVDR transition or will do so before July 2023.
Transitional timelines
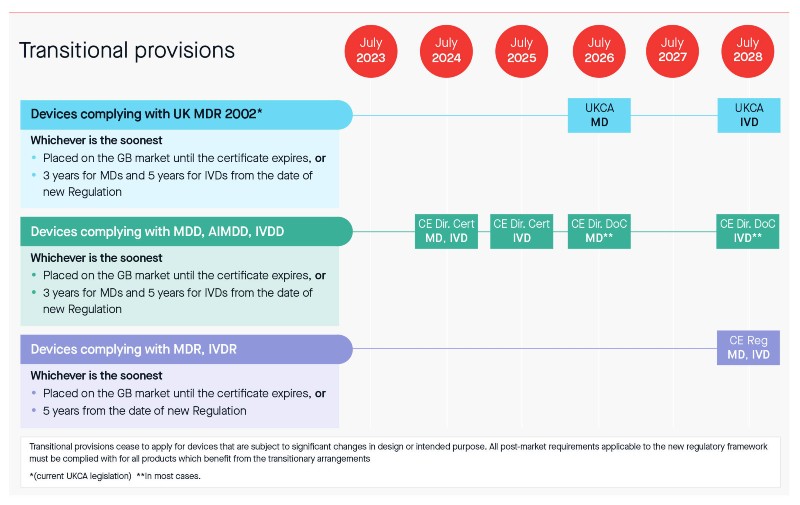
As manufacturers, you have the choice to decide which approach suits you best taking the following into consideration:
- The types of devices you have.
- Your Directive Certificate expiry dates.
- Your progress in transitioning towards MDR/IVDR.
- Your unique style of working.
BSI will continue to take UKCA applications under the current legislations on a case-by-case basis, please contact your BDM for more details. You can also find further information around UKCA legislation on our dedicated web page.
Whilst BSI cannot advise manufacturers on what they need to do, we are available to answer any of your questions regarding this transition. Please don’t hesitate to contact your scheme manager with any queries.
Yours sincerely,
Vishal Thakker
Head of UK Approved Body & Senior Regulatory Lead, Regulatory Services (Medical Devices)



