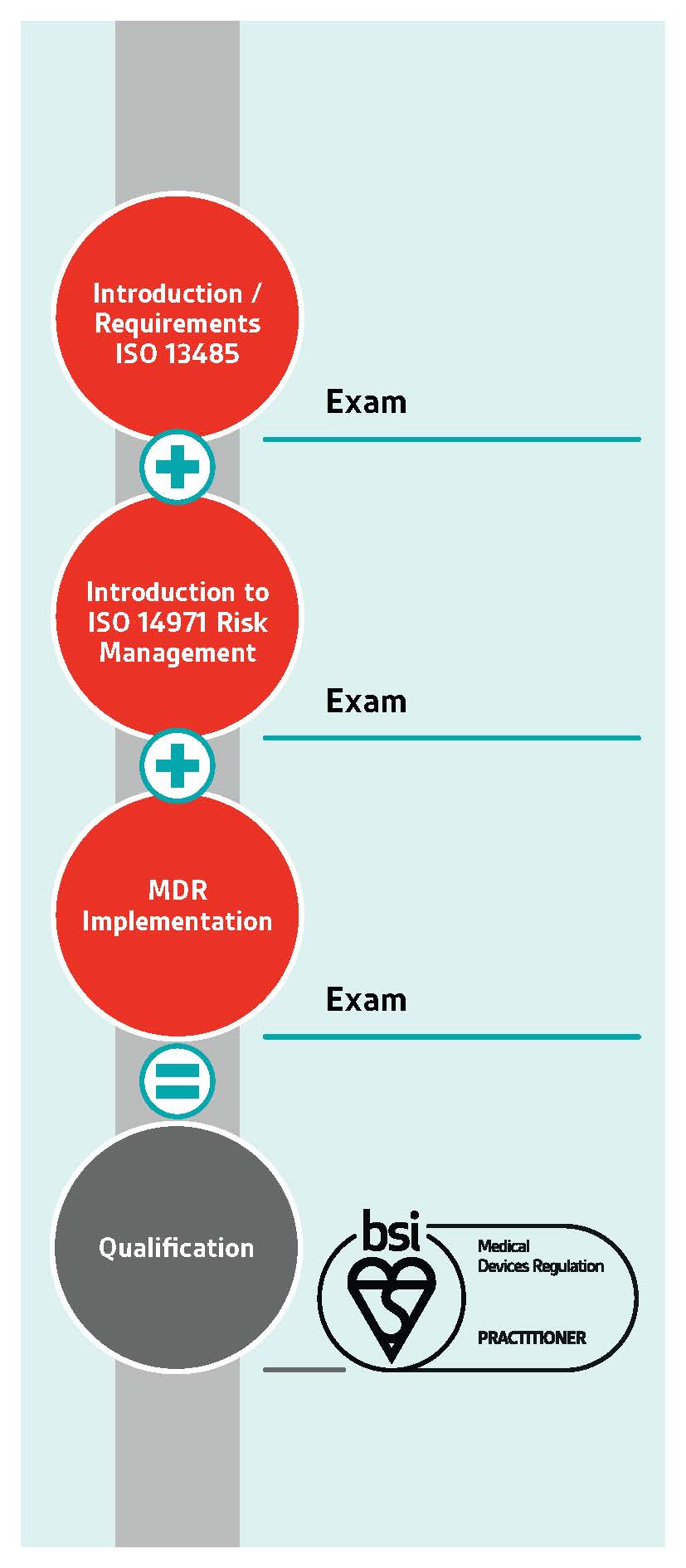
As a medical device professional, you understand the importance of implementation and maintenance of compliance to the Medical Devices Regulation (MDR). Central to this is the ability to demonstrate comprehensive, in-depth knowledge and the skills required to direct and guide an organization in all matters related to the quality and safety of the medical device throughout its lifecycle.
BSI’s interrelated qualifications consist of carefully selected training courses, delivered by industry experts, and underpinned by assessments. Upon successful completion, share your internationally recognized Mark of Trust, a formal recognition of your learning and knowledge, within your organization, with clients and your peers.